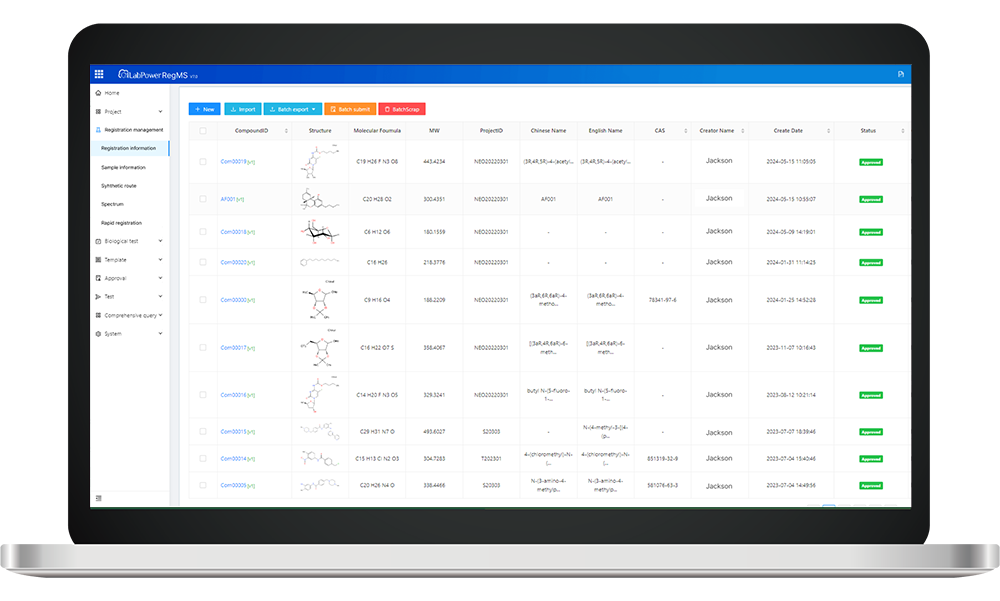
The global biotechnology market is projected to reach a staggering $727.1 billion by 2025, with antibody-based therapies playing a pivotal role in this growth. In Spain, the adoption of an antibody Registration system (ARS) has become increasingly vital as we navigate through complex regulatory landscapes and strive for innovation.
Key Features of Neotrident’s Antibody Registration System in Spain
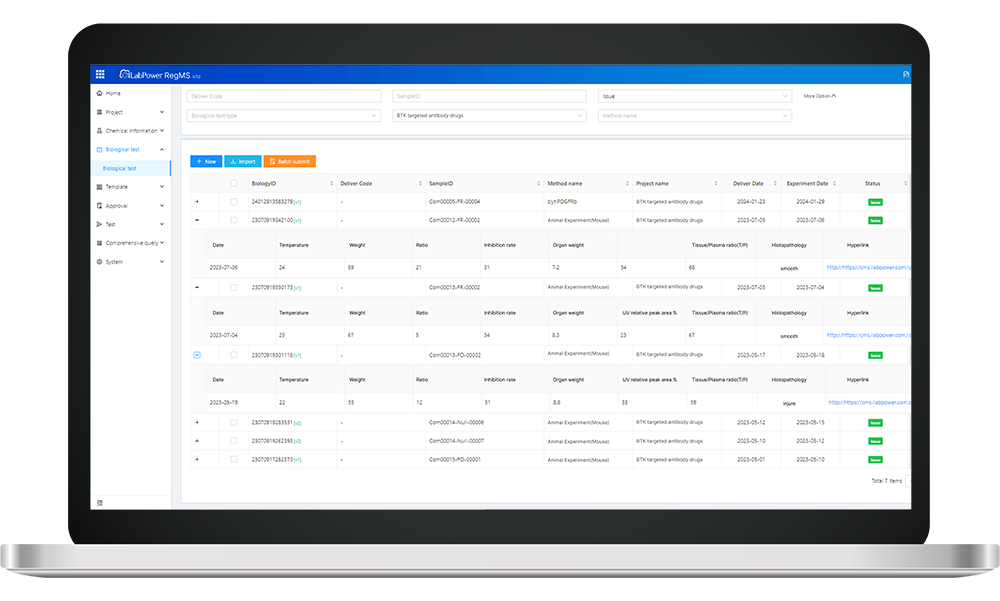
Neotrident stands out as a leader in implementing an effective Antibody Registration System within the Spanish biotech sector. Here are some defining characteristics:
Find more about R&D project management software.
- Streamlined Regulatory Compliance: Neotrident’s ARS ensures that all antibody products meet stringent EU regulations, facilitating smoother approvals and reducing time-to-market.
- User-Friendly Interface: The system boasts an intuitive design that allows researchers and manufacturers to easily register their antibodies, enhancing user experience and efficiency.
- Comprehensive Database Access: Users benefit from access to a robust database containing extensive information on existing antibodies, aiding in research and development efforts while minimizing redundancy.
- Sustainability Focus: Neotrident emphasizes eco-friendly practices within its registration process, aligning with Spain’s commitment to sustainability in healthcare solutions.
- Catalyst for Innovation: By simplifying the registration process, Neotrident fosters an environment conducive to innovation among biotech firms looking to develop new therapeutic options.
The Future Outlook of Antibody Registration Systems in Spain
The implementation of advanced systems like Neotrident’s ARS marks a significant shift towards more efficient biotechnological advancements in Spain. As we continue to witness rapid developments within this field, it is clear that such systems will play an integral role not only in compliance but also as catalysts for groundbreaking discoveries. With ongoing investments into biotechnology infrastructure and education, I am optimistic about the future trajectory of antibody registration processes across Europe—especially here at home in Spain.